Chapter contents:
Arthropoda
–– 1. Stem-group Arthropods
–– 2. Trilobita
–– 3. Chelicerata
–– 4. Mandibulata ←
The above image shows the large, toothed mandibles of a bull ant (Insecta). Mandibles are a unifying feature of the Mandibulata. Image by fir0002; Creative Commons Attribution-NonCommercial Unported 3.0 license.
Introduction
Mandibulata holds the distinction of being the most diverse and abundant group of animals on the planet. A large portion of this diversity is partitioned in the Hexapoda, which includes the insects. All mandibulates are united by a pair of post-oral, food processing appendages (see image below), called mandibles. For comparison, this same set of appendages develops into a pair of walking legs in the Chelicerata (the other major arthropod group; learn more on the Chelicerata page). In turn, the chelicerae appendages, for which chelicerates are named, develop into sensory appendages (i.e., antennae) in mandibulates. Based on molecular data, these two groups, Mandibulata and Chelicerata, likely diverged during the Ediacaran, more than 540 million years ago.

A generalized body plan of a malacostracan (i.e., crabs, shrimp, lobsters) mandibulate. Note the antennae and mandibula, which distinguish mandibulates from chelicerates. Learn more about the Malacosstraca below. Image by Hans Hillewaert; Creative Commons Attribution-Share Alike 4.0 International license.
As shown in the phylogeny below, mandibulates are split into two major groups, Myriapoda and Pancrustacea (see below for more on each of these groups). Myriapoda includes familiar animals like millipedes and centipedes, and their relatives. Pancrustacea is a much more diverse group in terms of morphology and number of species. Considering the number of species, a majority of this prolific diversity is within the Insecta. In addition to the broad morphological diversity of insects, the crustacean—a paraphyletic but commonly used grouping—show a range of body plans and ecologies. A variety of the pancrustacea, such as the clades including barnacles, copepods, crabs, and insects, are introduced in the section below.
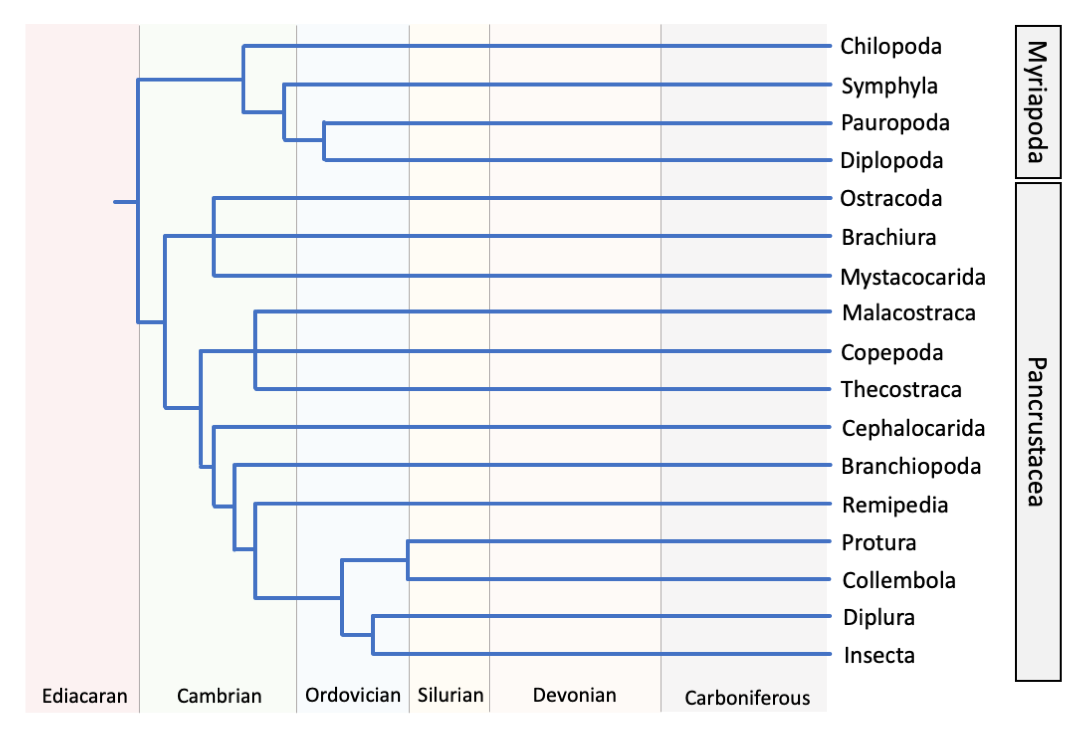
A hypothesis on the phylogenetic relationship of extant clades within the Mandibulata. Redrawn from Figure 3 in Giribet and Edgecombe (2019) in Current Biology.
Myriapoda
The myriapods are a monophyletic clade composed of four major groups, Chilopoda, Diplopoda, Symphyla, and Pauropoda. All known extant species are terrestrial, and breath using a trachea system. The diplopods, which include millipedes, are by far the most diverse, with more than 12,000 living species described. Diplopods, as suggested in their name (“diplo” = double; “poda” = feet), have two pairs of legs on each of their numerous body segments. Chilopoda includes another familiar group, centipedes, and are represented by at least 3,500 species. Notably, chilopods produce venom, which they use to subdue prey and to deter their own predators. The remaining groups, Symphyla and Pauropoda, are much less diverse, with approximately 200 and 850 species, respectively. Species of both Symphyla and Pauropoda live primarily in soil ecosystems where they consume detritus.

A red-leg centipede (Cormocephalus nitidus) from South Africa. Image by Joubert Heymans; Creative Commons Attribution-NonCommercial 4.0 International license.
As shown in the phylogeny above, the venomous, predatory Chilopoda are the outgroup to the remaining myriapods, with Symphyla branching basally to the Dignatha, which includes Diplopoda and Pauropoda. The relationships between the four myriapod groups are now generally accepted in this arrangement, though multiple hypotheses have been presented in the past. Studies on the evolution, and relationships between groups, has been hindered by a lack of stem-group fossils and a sparse fossil record during the early evolutionary history of myriapods. It has been suggested that the euthycarcinoids may be stem myriapods; however, as these were marine species and all known living myriapods are terrestrial, this claim has been met with speculation. The earliest accepted evidence of myriapods in the fossil record comes via trace fossils from the Late Ordovician (watch the video below to see what traces like these look like).
Learn more about the giant Carboniferous myriapoda, Arthropleura, in "First Life with David Attenborough-Giant Millipede" by Discovery (YouTube).
The myriapods have two notable distinctions: they are likely the world’s first and oldest land animals and include the largest terrestrial arthropods ever to have lived. As early terrestrial ecosystems developed during the Ordovician, myriapods are thought to have been the first animals to take advantage of the new ecological space. It remains to be seen whether myriapods colonized land once and evolved from a single, terrestrial ancestor; or, a diverse myriapod fauna evolved first and repeatedly transitioned to land. Later in the Paleozoic, during the Carboniferous, extremely high concentrations of oxygen in the atmosphere likely facilitated increased sizes in arthropods from this time. The myriapods in the genus Arthropleura are often considered to be the largest of these, reaching lengths of 2.5 meters (watch the video above to learn more).
Pancrustacea
Pancrustacea includes a variety of morphology diverse animals—like barnacles, crabs, insects, and ostracods—and are found in essentially all of Earth’s habitats. The group is also occasionally called Tetraconata, in reference to members of this group having four cone cells in each of their compound eyes. Pancrustacea, which is now broadly accepted as a monophyletic group, is used in place of Crustacea because Crustacea was a paraphyletic group, with respect to the phylogenetic position of Hexapoda (including the insects). Outside of a phylogenetic context, crustacea is still commonly used to refer to shelled animals.
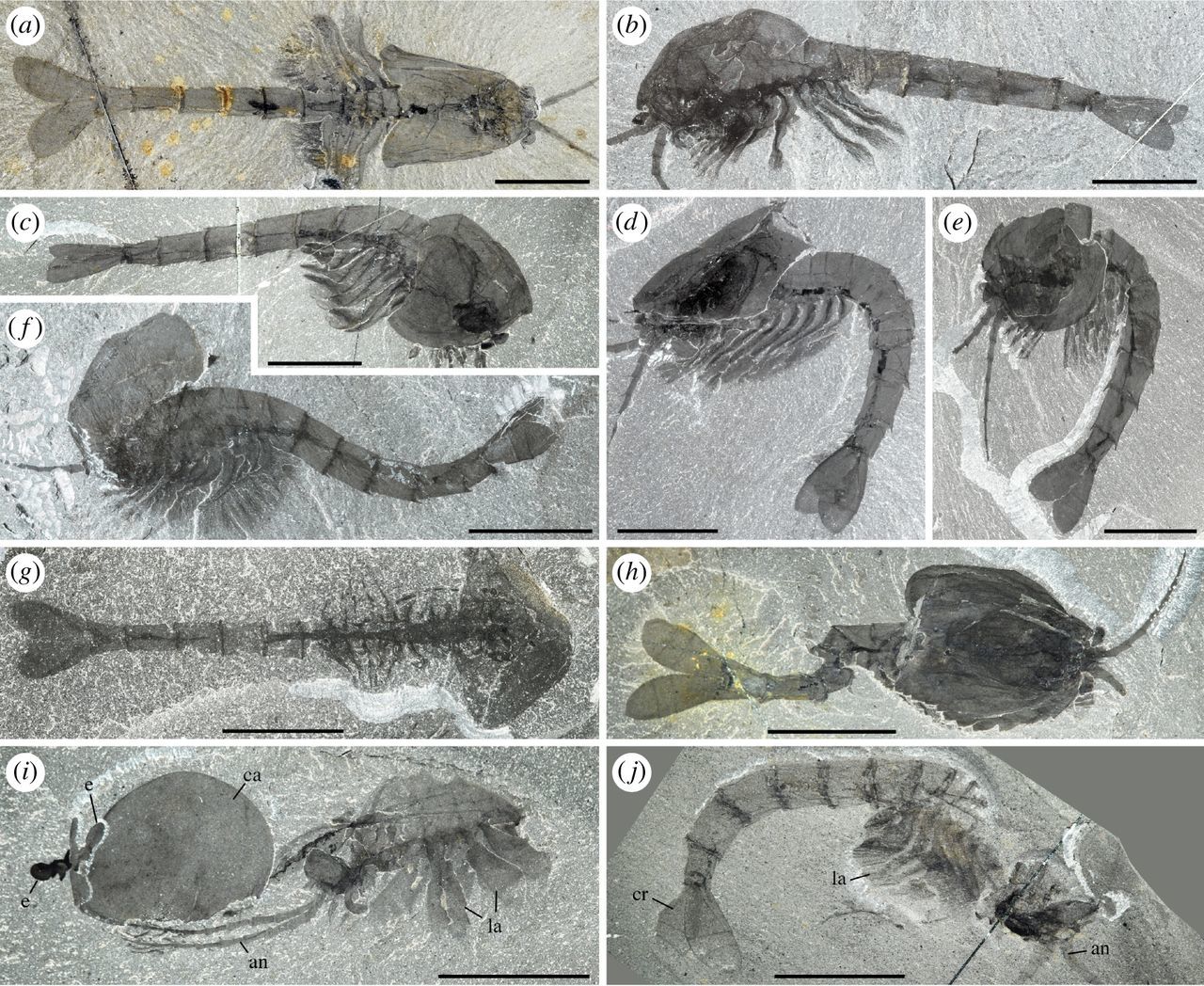
"Waptia fieldensis Walcott, 1912 [10] from the middle Cambrian (Series 3, Stage 5) Burgess Shale, British Columbia, Canada; preservational aspects. (a,b) USNM 57681a, USNM 139214; posterior part of body with a straight profile in dorsal and lateral views, respectively. (c–f) ROMIP 56421, USNM 268270, ROMIP 64281, USNM 529131, body with slightly curved, recurved and sigmoidal profile in lateral view. (g) ROMIP 64282 with carapace tilted downwards and most swimming (lamellate) appendages decayed. (h) ROMIP 64283, with broken abdominal region. (i) ROMIP 56427, specimen with disarticulated cephalic elements (carapace detached from the rest of the body; see also figure 6). (j) ROMIP 64284 with the anterior part detached from the rest of the body. All images are photographs taken under cross-polarized light. Abbreviations are as follows: an, antennule; ca, carapace; cr, caudal ramus; e, eye; la, lamellate post-cephalothoracic appendage. Scale bars: 1 cm." Figure and caption from Vannier et al. (2018) in Royal Society Open Science; Creative Commons Attribution 4.0 International license.
Representatives of Pancrustacea are known from the Cambrian onward (see Waptia above), including crown-group members from at least 518 million years ago. As mentioned above, one of the common features of Pancrustacea is the presence of four cones in each ommatidia—the individual eyes making up the compound eye. Other similarities within Pancrustacea are related to soft-tissue features, patterning of appendages, and segments of the body. Though there is agreement on the monophyly of Pancrustacea, the evolutionary relationships between groups remains an area of active research, spurred on by the incredible diversity and complexity of Pancrustacea. In the sections that follow, a subset of the more familiar Pancrustacea are introduced, highlighting the ecological and morphological diversity of this group.
Ostracoda
Ostracods are small, two-valved arthropods that first appeared in the fossil record during the Ordovician, approximately 485 million years ago. More than 65,000 fossil and living species have been described. Originally a marine clade, modern species in this class are known from a broad range of habitats, from leaf litter in forest ecosystems to lakes to ocean depths of 7000 meters, and just about everywhere in between. Though they are small in size—usually under 2 millimeters and occasionally as large as a few centimeters—ostracods are the most abundant arthropods in the fossil record. Remarkably, the general ostracod body plan has changed very little during the nearly 500-million-year evolutionary history of this class. As highlight by Williams et al. (2015), fossilized ostracods with soft tissues preserved, from the Ordovician and Silurian, are morphologically similar to those living today (watch the video below to see living ostracods).
Check out these living ostracods in this video, "Ostracods under the microscope," by Sci-Inspi (YouTube).
One of the factors contributing the persistence and resilience of ostracods is their tolerance of most abiotic conditions. As reviewed by Williams et al. (2015), these tiny arthropods can handle a wide range of conditions—and have even been taken to space! For example, the species Candona candida can survive in a pH range of 5.3 to 13, and other species have been found in waters with a pH as low as 3.4—highly acidic conditions. Likewise, ostracods can be found in polar waters as cold as 0° C and in hot springs warmer than 50° C. The capacity to adapt to these various conditions has allowed ostracods to thrive. Additionally, ostracods employ multiple reproductive strategies. Many species brood their young, keeping their eggs and juveniles within the protective shell of the adult until they are fully developed. Other species, particularly those in freshwater habitats, deposit eggs into the sediment, where they are protected from predators. Though most species reproduce sexually, with contributions from a sperm-donor and egg-donor, many freshwater species are capable of parthenogenesis. In parthenogenesis, the egg-donor essentially clones itself.
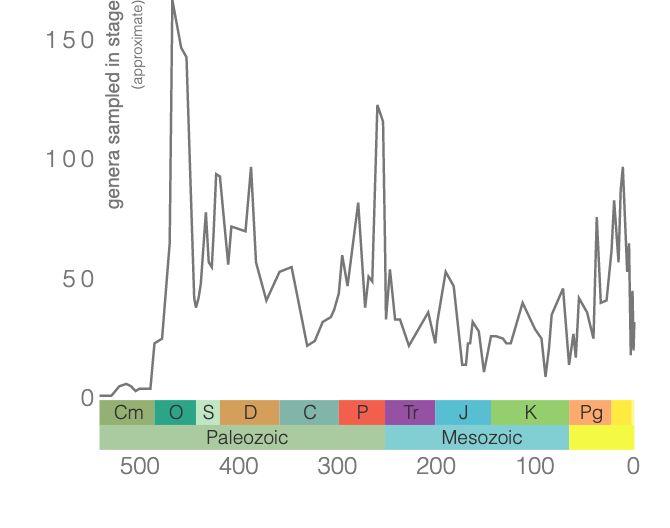
Diversity of ostracod genera through time. Figure generated using the Paleobiology Database Navigator.
The fossil record of ostracods begins approximately 485 million years ago, during the Ordovician. Early in their evolutionary history, ostracods lived primarily in ocean bottom habitats. Because of their abundance and broad distributions in space and time, ostracods are often used in biostratigraphy (i.e., are used to correlate ages and relationships of formations in the rock record). During the Silurian, ostracods radiated and evolved to thrive in the plankton. Reflecting their ecologies, ostracods have two major groups: myodocopes and podocopes. The myodocopes include those ostracods that radiated into planktonic forms and the podocopes are exclusively benthic. During the middle of the Paleozoic, podocopes began to radiate into freshwater habitats and, by the end of the Carboniferous, were well established in lake and pond habitats. Evidence of at least seven marine-to-freshwater colonization events are preserved in the fossil record.
Limestone rock covered by numerous ostracods and other fossil debris; specimen is from the Ordovician of Lafayette County, Wisconsin (PRI 76750). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Longest dimension of rock is approximately 15 cm. Model by Emily Hauf.
Malacostraca
Malacostraca includes many of the taxa commonly thought of as crustaceans, like crabs, lobsters, and shrimp. These familiar taxa all belong to the same order, Decapoda, which is one of several living and fossil orders of malacostracan. Malacostraca also includes mantis shrimp (order Stomatopoda), isopods (superorder Peracarida), krill (order Euphausiacea), and a variety of other groups. Represented by at least 40,000 living species, malacostracans characteristically have three major body regions, head, thorax, and abdomen, plus a telson. With few exceptions (e.g., Remipedia), the malacostracans have 20 body segments, with five in the head, eight in the thorax, six in the abdomen, and the telson. These crustaceans are most abundant in marine habitats, where they are largely scavengers and predators, but can also be found in many freshwater and some terrestrial habitats. As discussed by Hegna et al. (2020), the first malacostracans in the fossil record are from the Cambrian and belong to the subclass Phyllocarida, which is represented today by a single order, Leptostraca. Phyllocarids were, and still are, small marine bivalved arthopods (see Nebalia in the figure below).

A selection of living malacostracan taxa. Left: A leptostracan, Nebalia, by Hans Hillewaert; Creative Commons Attribution-Share Alike 4.0 International license. Middle: An isopod, Armadillidium, by Katja Schulz; Creative Commons Attribution 4.0 International license. Right: A stomatopodan, Odontodactylus, by Mark Rosenstein; Creative Commons Attribution 4.0 International license.
Decapods—meaning “ten feet”—are prominent, familiar members of the Malacostraca and have played an important role in ecosystems since at least the Mesozoic. As reviewed by Hegna et al. (2020), the earliest decapods in the fossil record are from the Late Devonian (~370 – 360 million years ago). Though they would become abundant starting during the Triassic, decapods are rare in the Paleozoic record. Represented by a diversity of species resembling modern shrimp and lobsters, the decapods appear to have radiated at during the Triassic—a lack of fossils from the end of the Paleozoic means there was possibly a longer, more complex evolutionary history. It was not until the Jurassic that modern crabs (i.e., Brachyura) became abundant; however, the incomplete early record of this group means, again, an earlier, longer evolutionary history is possible. Regardless, the ecological importance of decapods became pronounced during this time. They were among the early, powerful crushing predators, which contributed to increased predation and competition in marine ecosystems during the Mesozoic (i.e., Marine Mesozoic Revolution).
Explore a few malacostracans in the 3D models below.
Modern specimen of the crab Hepatus epheliticus (locality information unavailable). Specimen is from the teaching collections of the Paleontological Research Institution, Ithaca, New York. Width of specimen is approximately 10 cm. Model by Emily Hauf.
Fossil specimen of the ghost crab Ocytode quadrata from the Pleistocene Anastasia Formation of Brevard County, Florida (PRI 43014). Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Specimen is approximately 15 cm in width. Model by Emily Hauf.
Fossil specimen of the shrimp-like crustacean Belotelson magister from the Pennsylvanian Mazon Creek lagerstätte of Will County, Illinois. Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Specimen is preserved inside a concretion. Length of specimen (not including surrounding rock) is approximately 4.5 cm. Model by Emily Hauf.
Thecostraca
Thecostraca is a unique group in Pancrustacea in that the adult forms in this group are all sessile, either as parasites to other animals or as suspension feeders (see image below for an example of a thecostracan life cycle). Barnacles (i.e., Thoracica) are an example of the latter and are the most well-known, and common, of the thecostracans (see 3D model below). In addition to the Thoracica (~1000 species), there are four other groups in Thecostraca: Facetotecta (~10 species), Ascothoracida (~90 species), Acrothoracia (~80 species), and Rhizocephala (~250 species). Facetotecta are an enigmatic, extant group that is currently only known in its juvenile form; no adult specimens have been found and it is suspected that they are endoparasites of other marine animals. Though discovery of adult forms may change their positioning, Pérez-Losada et al. (2012) found Facetotecta to be the sister group to the other four groups. Ascothoracida are also accepted to be the sister group to the remaining three groups, which are collectively referred to as Cirripedia. In the broader Mandibulata phylogeny, Thecostraca are commonly grouped with Copepoda and Malacostracn to form the superclass Multicrustacea.
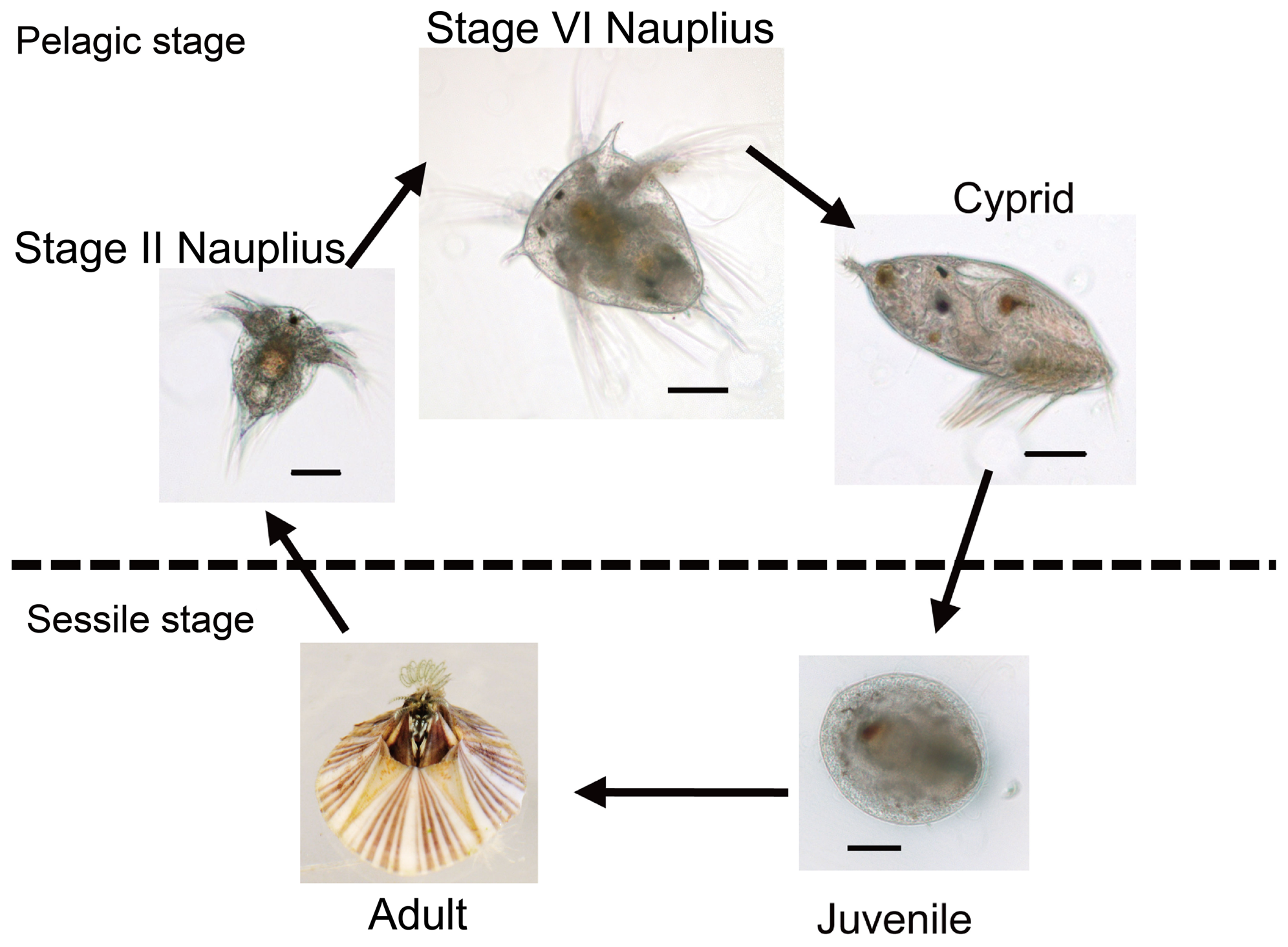
A simplified version of the barnacle life cycle, showing the sessile and pelagic stages. "Developmental stages of the barnacle Balanus amphitrite that were sampled for transcriptomic and gene expression analysis. The stages include stage II nauplius, stage VI nauplius, cyprid, juvenile and adult. The larval cDNA pool consisted of stage II nauplius, stage VI nauplius and cyprid, while the adult cDNA pool was staged only. The diameter of the adult barnacle is ∼2 cm. Scale bars = 100 µm." Figure and caption from Chen et al. (2011) in PLoS ONE; Creative Commons Attribution 4.0 International license.
The fossil record of Thecostraca is dominated by barnacles (i.e., Thoracica), as they are the only group with a robust, calcium carbonate skeleton. Yet, as reviewed by Hegna et al. (2020), there is speculative evidence of the other groups in the fossil record. Ascothoracids from the Cretaceous Period have been inferred based on the presence of cysts and trace fossils in other animals, similar to found on ascothoracid hosts today. Similarly, acrothoracican have been inferred based on small, slit-like traces from the Devonian Period. Evidence of rhizocephalans is, likewise, limited. Living rhizocephalans castrate the male crabs they parasitize, resulting in a observable morphological changes in the male crab. Crabs exhibiting these morphological changes have been observed in Miocene and Cretaceous specimens. As discussed by Klompmaker and Boxshall (2015), who studied the Miocene specimens, the morphological changes are known to occur for other reasons, leaving open the possibility that rhizocephalans were not responsible, or even present. The earliest thoracicans in the fossil record are from the Silurian, with the first consensus crown-group species occurring as early as the Carboniferous. Check out fossil barnacles from the Pliocene in the 3D model below.
Fossil barnacles Balanus sp. that have encrusted the shell of a gastropod (Hysterivasum sp.); specimen is from the Pliocene Tamiami Fm. (Pinecrest Beds) of Sarasota County, Florida. Specimen is from the collections of the Paleontological Research Institution, Ithaca, New York. Length of specimen is approximately 7 cm. Model by Emily Hauf.
Some living and fossil barnacles attach(ed) to large, mobile animals, like turtles and whales. As a consequence, the calcium carbonate skeleton of the barnacles can be analyzed to recreate movement patterns of the larger animals because composition of the barnacle skeleton reflects the water chemistry where it grew. This mode of life, epibiont attachment, likely evolved during the Cenozoic, as there is not any evidence of barnacles on large animals from the Mesozoic, or earlier. Watch the video below to learn more about this unique mode of life!
Learn more about what barnacles can tell us about the whales they sometimes attach to in "A whale of a ride?" by National Science Foundation (YouTube).
Insecta
Insects are ubiquitous on Earth. To date, more than one million insect species have been described and it is generally accepted that several million more await formal scientific discovery and description. Species in this class can be found in essentially all terrestrial habitats—plus some marine habitats—and exhibit a wide range of morphologies. A typical insect has an exoskeleton made of chitin, three main body regions (head, thorax, abdomen), three pairs of legs (as in all hexapods), and a single pair of antennae. You can see many of these features in the fossil dragonfly below, and the artistic reconstruction.

A fossil dragonfly from the Carboniferous and a reconstruction of a closely related species, from the same genus. Left image caption: "Meganeurula selysii (Brongniart, 1893), holotype MNHN R52939, head and prothorax. Ventral view showing the false ‘mandibles’ carved in matrix (photograph Gaelle Doitteau, e-recolnat Project, MNHN). Scale bar, 10 mm." Right image caption: "Meganeurites gracilipes Handlirsch, 1919, reconstruction. Pattern of coloration highly hypothetical, adapted from extant relatives, caudal appendages of abdomen corresponding to Namurotypus sippeli (reconstruction M.P.). Scale bar, 10 mm." Images and captions from Nel et al. (2018) in Science Reports; Creative Commons Attribution 4.0 International license.
As shown in the phylogeny at the beginning of this Mandibulata page, Insecta is most closely related to the Diplura, Collembola, and Protura, forming a strongly supported clade called Hexapoda—referring to the six legs common to the body plans of these groups. Until the development of advanced molecular phylogenetic techniques, hexapods were thought to be most closely related to the myriapods. In particular, the tracheal respiratory system found in both groups made a compelling argument for this close relationship. With Hexapoda and Myriapoda forming a clade, a monophyletic Crustacea was considered to be the sister group. Molecular phylogenetics have provided strong evidence for the placement of Hexapoda within a paraphyletic Crustacea. This latter placement of Hexapoda is now widely accepted, as part of the clade Pancrustacea, with Myriapoda as a sister group. In a sense, insects can be considered terrestrial crustaceans.

Fossil insects from the Cretaceous preserved in amber. "AMBER No. 01 with the specimens of Mesophthirus engeli Gao, Shih, Rasnitsyn & Ren, gen. et sp. nov. from the mid-Cretaceous of Myanmar. a Photo of the whole feather and the locations of the insects, corresponding to the Supplementary Fig. 1. b–i Paratypic specimens CNU-MA2016001 to CNUMA2016008. j Holotypic specimen CNU-MA2016009. k Parts of the feather show complete areas at basal part and adjacent largely damaged area between barbs. Representative, star in white referring to relatively complete barbules, star in blank referring to large areas of damages. Scale bars, 1mm (a), 100 μm (b–j), and 0.5mm (k)." Figure and caption from Gao et al. (2019) in Nature Communications; Creative Commons Attribution 4.0 International license.
The first insects in the fossil record are from the Rynie Chert (Scotland) and lived during the Devonian, approximately 411 million years ago. The insect fauna of the Devonian was already diverse, with some species showing derived features, including traits associated with flight in modern insects. As such, it is likely that the evolutionary history of Insecta extends at least to the Silurian—a time when other arthropods were transitioning to land and diversifying. Interestingly, insects, and other hexapods, are almost completely absent from the fossil record for roughly 60 million years at the end of the Devonian and beginning of the Carboniferous, a feature known as the “hexapod gap.” In addition to the transition to land, the evolution of flight in Insecta was a major evolutionary advancement. As discussed in the video below, the timing of this evolution remains uncertain. Some have argued that flight had already evolved during the Devonian, whereas others more conservatively suggest the innovation occurred during the Carboniferous, as indicated by fossilized insect wings from this time. The answer to this question may lie in the hexapod gap, necessitating further study. Regardless of when flight evolved, it contributed substantially to the evolutionary success of insects, as it likely freed them from many of their predators and enabled them to utilize previously unobtainable resources. Insects flourished during the Carboniferous, when extremely high oxygen concentrations in the atmosphere enabled them to reach large size (see the dragonfly, Meganeurula, above for an example). Though they experienced some extinction during the end-Permian event, insects flourished again during the Mesozoic and began to resemble the modern insect fauna during this time. As evidenced by their continuing vast abundance and diversity today, insects are undoubtedly one of the most successful groups to have ever lived.
Learn more about the evolution of flight in insects and its role in their abundance today in "When Insects First Flew" by PBS Eons (YouTube).
References and Further Reading
Aria, C. and J. B. Caron. 2017. Burgess Shale fossils illustrate the origin of the mandibulate body plan. Nature, 545: 89-92.
Engel, M. S. 2015. Insect evolution. Current Biology, 25: R868-R872.
Gao, T., X. Yin, C. Shih, A. P. Rasnitsyn, X. Xu, S. Chen, et al. 2019. New insects feeding on dinosaur feathers in mid-Cretaceous amber. Nature Communications, 10: 1-7.
Giribet, G., and G. D. Edgecombe. 2019. The phylogeny and evolutionary history of arthropods. Current Biology, 29: R592-R602.
Grimaldi, D. A. 2010. 400 million years on six legs: On the origin and early evolution of Hexapoda. Arthropod Structure & Development, 39: 191-203.
Hegna, T. A., J. Luque, and J. M. Wolfe. 2020. The fossil record of the Pancrustacea. The Natural History of the Crustacea: Evolution and Biogeography of the Crustacea, Volume 8, 21.
Jain, S. 2020. Ostracods. In Fundamentals of Invertebrate Palaeontology (pp. 143-170). Springer, New Delhi.
Klompmaker, A. A. and G. A. Boxshall. 2015. Fossil crustaceans as parasites and hosts. Advances in Parasitology, 90: 233-289.
Misof, B., S. Liu, K. Meusemann, R. S. Peters, A. Donath, C. Mayer, et al. 2014. Phylogenomics resolves the timing and pattern of insect evolution. Science, 346: 763-767.
Nel, A., J. Prokop, M. Pecharová, M. S. Engel, and R. Garrouste. 2018. Palaeozoic giant dragonflies were hawker predators. Scientific Reports, 8: 1-5.
Pérez-Losada, M., J. T. Høeg, and K. A. Crandall. 2012. Deep phylogeny and character evolution in Thecostraca (Crustacea: Maxillopoda). Integrative and Comparative Biology, 52: 430-442.
Schwentner, M., S. Richter, D. C. Rogers, and G. Giribet. 2018. Tetraconatan phylogeny with special focus on Malacostraca and Branchiopoda: highlighting the strength of taxon-specific matrices in phylogenomics. Proceedings of the Royal Society B: Biological Sciences, 285: 20181524.
Vannier, J., C. Aria, R. S. Taylor, and J. B. Caron. 2018. Waptia fieldensis Walcott, a mandibulate arthropod from the middle Cambrian Burgess Shale. Royal Society Open Science, 5: 172206.
Williams, M., V. Perrier, C. Bennett, T. Hearing, C. Stocker, and T. Harvey. 2015. Ostracods: The ultimate survivors. Geology Today, 31: 193-200.
Zhai, D., J. Ortega-Hernández, J. M. Wolfe, X. Hou, C. Cao, and Y. Liu. 2019. Three-dimensionally preserved appendages in an early Cambrian stem-group pancrustacean. Current Biology, 29: 171-177.
Usage
Unless otherwise indicated, the written and visual content on this page is licensed under a Creative Commons Attribution-NonCommercial-Share Alike 4.0 International License. This page was written by Jansen A. Smith. See captions of individual images for attributions. See original source material for licenses associated with video and/or 3D model content.




